The Life System
Package I
* One LIFE System device and one license.
* One free, fully-loaded laptop.
* One free Body Balancer.
* One set of harness straps.
* FREE Basic Training with a Certified Instructor.
* 3 years of free Technical Support.
* Free 4-wheeled carrying case for travel.
Package II
* One LIFE System device and two licenses.
* Two Body Balancers and two licenses.
* One Free HEBII.
* Two free, fully-loaded laptops.
* Two sets of harness straps.
* FREE Basic Training with a Certified Instructor.
* 3 years of free Technical Support.
* Free 4-wheeled carrying case for travel.
HOW TEST MEASUREMENTS ARE MADE
Information exchange is available via the harness system attached to the forehead, ankles and wrists. The harnesses are made of special conductive silicone, which allows for very low- current electrical communication through the computer’s USB port and a multi-functional interface box.
During the testing/assessment phase a client’s biofeedback response is measured in response to nearly 7,000 separate items in 39 separate categories. This process takes approximately 4 to 7 minutes depending upon the speed of the computer. The computer calculates an average value of response between 0 and 2000, which results in a list defining the individual’s reactions or stress potentials. The greatest energy disturbances will be recorded and displayed at the top of the list. Each substance has its own distinctive, complex waveform, which is graphically viewed and represented by a unique fractal image. Additionally, the test matrix contains a detailed description of each substance.
HOW THE L.I.F.E. BIOFEEDBACK SYSTEM WORKS
L.I.F.E. System – Technical Information
The measurement made by the L.I.F.E. System is based upon the relationship between “action & reaction” by applying a challenge to the patient (“action”) and measuring the reaction of the human body as it answers the challenge (“reaction”).
The basic principle of the mode of action of the L.I.F.E. System device is the following: The L.I.F.E. System device sends a square wave signal with amplitude of 5 volts, and a duty cycle of 50% to the harness. The frequency to be applied, for determination of our test, is about 47.3 kHz. The measurement current throughout the body is limited to a maximum of 10mA, but usually no more than 5mA or less.
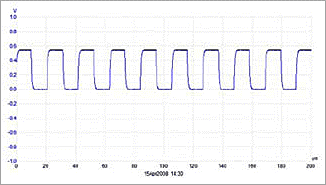
Figure 1 shows the applied signal, without the harness being in contact with the human body.
Figure 1: Square wave signal with no harness in contact with the human body
When the harness is in contact with the human body, a significant change (deformation) of the signal can be detected. Figure 2 shows the respective signals (but on a different time scale to underline the changes)
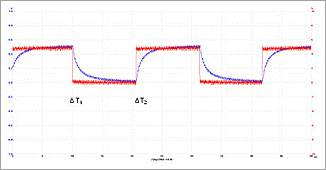
Figure 2: Superimposition of the signal without harness being applied to the human body (red signal) and with electrodes being applied to the human body (blue signal).
As can be seen from the superimposition of the signals, there is a significant difference in the shapes of the signals. The detection of the signal is realized by the L.I.F.E. System device in a binary way – the part of the signal being placed in the upper half of the square wave signal is detected, the lower part of the square wave signal is ignored. Therefore, the different shapes of the signals (electrodes applied to a patient and no electrodes applied to the patient) result in time differences ΔT1 and ΔT2 before half of the amplitude is passed. These time differences resulting from the different shapes of the signals are detected by the L.I.F.E. System device.
When applying the same methodology to a patient suffering from a particular imbalance, again a signal will be detected. But as the situation in the body of the patient is different due to the imbalance, the shape of the signal to be detected is different, and it can be seen that this signal is less steep in the case of imbalance. This difference in signal shapes results in other time differences Δt1 and Δt2, which are characteristic for a particular imbalance.
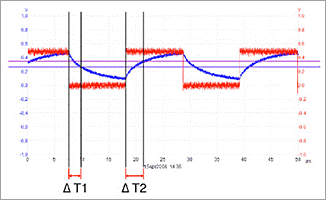
Figure 3 shows the signal obtained in the case of such an imbalance
Figure 3: Superimposition of the Signal obtained from a patient suffering from a particular imbalance (blue signal) and the signal obtained when harness is not applied to the human body (red signal)
However, it must be pointed out that the results obtained by the L.I.F.E. System device can only give hints to physical states in which the presence of a particular imbalance is likely. The final diagnosis must be confirmed by other methodologies such as X-ray or MRI in any case.
The basic principle of the L.I.F.E. System device is to measure these time differences of the respective signals and to achieve a statistical evaluation based on a mathematical algorithm. The results obtained are compared with data which are archived in a data base.
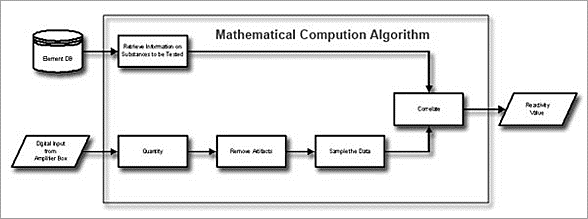
Figure 4: Basic work flow for the evaluation of data obtained
The mathematical calculation algorithm takes input information on the one hand, which is stored in the Element database, and on the other hand the digitized measurement data, belonging to the element. After quantifying the values, artefacts (like disturbances caused by EMC, movements, etc.) are removed. The cleaned data is sampled, and after that, the correlation is calculated. This leads to the desired reactivity value providing the results of reactivity testing relative to the given element.
It can be claimed that when the situation inside the human body is disturbed due to a particular imbalance, the physical situation will become different. Any abnormal condition can be considered as a kind of imbalance, it can be easily seen that the temperature inside the area of interest is different. As a consequence, the shape of the signal that is detected will differ from the one measured in a non-conditional situation. The differences can be measured easily by detecting the time difference, as described above, and those time differences are characteristic for the respective situations. Evidence that they can be measured and detected was given by the outcome of the clinical investigation.
Literature describing the effectiveness of the L.I.F.E. System device.
Literature in peer reviewed journals describing the mode of action and effectiveness of the L.I.F.E. System was not available prior to the development of the L.I.F.E. System. Consequently, a clinical investigation giving evidence to the effectiveness was undertaken in 2006. The complete documentation and the results had already been submitted to and approved for European Class 2-A Certification.
